Pharma: Page 3
-
What should pharma make of the FDA going dark on DEI?
After a series of potent executive orders last week, the FDA removed draft guidance on diversity in clinical trials.
By Meagan Parrish • Feb. 4, 2025 -
Drugmakers prep for bird flu outbreak, despite continued low risk
While the virus hasn’t made a sustained leap into humans, vaccines and treatments are coming through the pipes if it does.
By Kelly Bilodeau • Feb. 3, 2025 -
 Explore the Trendline➔
Explore the Trendline➔
 Stock via Getty Images
Stock via Getty Images Trendline
TrendlineClinical trial diversity
As pharma wises up to the fact that the current playbook for improving clinical trial diversity has yet to make a meaningful impact, the quest is on to refine that approach.
By PharmaVoice staff -
Deep Dive
A new, non-opioid pain drug is here. Getting it to patients could be agony.
After decades of research, Vertex Pharmaceuticals now has an approved pain medication. Can one of the world’s most powerful biotechs contend with a healthcare system that’s long favored opioids?
By Jacob Bell • Feb. 3, 2025 -
Year in Preview
Pharma’s forecast for 2025: Sowing seeds of a rebound
Despite regulatory uncertainty, pharma is bouncing back from a market slump and is being fueled by innovation.
By Meagan Parrish • Jan. 31, 2025 -
Is federal funding frozen? Has it already thawed? Flip-flop leaves biopharma on edge
NIH grants that help fund drug development were caught in the mix of confusing executive orders this week.
By Amy Baxter • Jan. 31, 2025 -
RFK Jr. faces reconciliation of past vaccine statements at Senate HHS hearing
“Are you supportive of these onesies?” Sen. Bernie Sanders asked Kennedy, pointing to infant clothing stamped with anti-vaccine slogans — and more from yesterday’s confirmation hearing.
By Michael Gibney • Jan. 30, 2025 -
A long-awaited alternative to opioids is on the FDA doorstep. Can Vertex seal the deal?
Vertex Pharmaceuticals’ non-opioid pain medication is up for FDA approval this week. Other drugmakers have found safer alternatives to expand the pain management landscape.
By Amy Baxter • Updated Feb. 4, 2025 -
Executive hiring changes reflect a broader biotech comeback
Executive hiring shifts are often indicators of wider trends in the market. Here’s how the hiring landscape for pharma leaders is changing.
By Alexandra Pecci • Jan. 29, 2025 -
Bringing drugs to market has ‘never been more challenging,’ and pharma is learning to adapt
As changes to the U.S. regulatory foundation take hold, pharmas need to change their strategies to keep innovation — and the bottom line — above the surface.
By Michael Gibney • Jan. 28, 2025 -
Q&A // First 90 Days
An ALS treatment wave is approaching, and this biotech CEO is ready to surf
Coya Therapeutics is entering into a phase 2b study for its ALS, and the biotech’s CEO believes it’s on the cusp of a game changer.
By Amy Baxter • Jan. 27, 2025 -
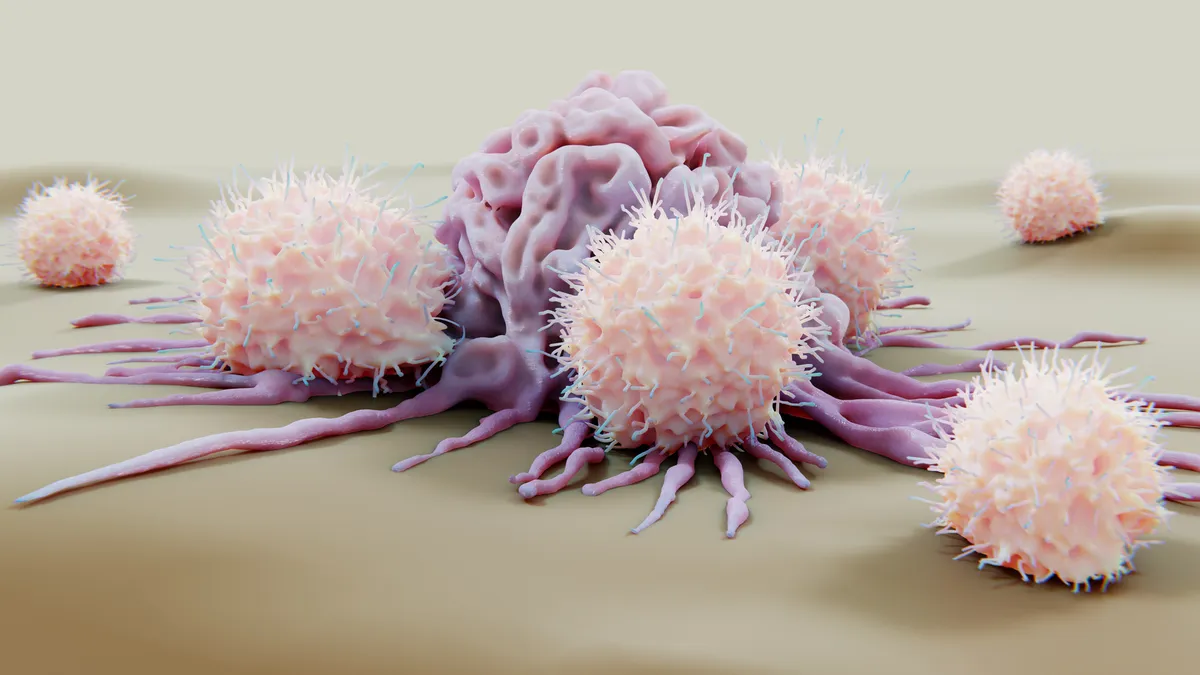
Natural killer cells, a rising alternative to CAR-T cell therapy
These innate immune cells may provide a safer, lower-cost option in cancer and autoimmune diseases.
By Kelly Bilodeau • Jan. 27, 2025 -
Gene therapies have been uneven for DMD — but these companies hope to turn the tide
Despite mixed results using gene therapies to treat Duchenne muscular dystrophy, drug developers are pushing ahead with the belief the answer could come down to delivery.
By Meagan Parrish • Jan. 24, 2025 -
J&J is all in on tau-targeted Alzheimer’s candidates. Will the approach lead to more effective treatments?
Anti-tau candidates haven’t crossed the regulatory threshold like their anti-amyloid counterparts, but J&J’s clinical efforts could lead in the right direction.
By Michael Gibney • Jan. 23, 2025 -
After an obesity stumble, BioAge reconnects with its longevity pipeline
The company is kicking off the year with a new Novartis partnership and renewed focus on metabolic aging targets.
By Alexandra Pecci • Jan. 22, 2025 -
Innovate or perish: Industry’s evolution leaves little room for me-too approaches
Novel drug approvals have set the stage for more breakthroughs in pharma.
By Amy Baxter • Updated Jan. 22, 2025 -
In pharma’s competitive climate, companies need evidence beyond clinical trials
Early planning and market research are crucial in an era of rising cost pressures.
By Kelly Bilodeau • Jan. 21, 2025 -
Deep Dive
‘The bar has risen’: China’s biotech gains push US companies to adapt
Pharma dealmaking for drugs invented in China is putting pressure on U.S. biotechs to compete harder.
By Ben Fidler • Jan. 17, 2025 -
Musk’s rare disease torpedo, hospitals’ pharma fix and other policies leaders are tracking
There’s more to pharma's regulatory story in 2025 than just the IRA.
By Meagan Parrish • Jan. 17, 2025 -
By the numbers: J&J, GSK lead J.P. Morgan deal announcements in 2025, the largest turnout in years
The J.P. Morgan Healthcare Conference has been a focal point for M&A year after year, and after a quiet period, the deals are back on the table.
By Michael Gibney • Jan. 16, 2025 -
What to expect in the sequel to Medicare’s drug price negotiation program
CMS is expected to publish up to 15 drugs for the second cycle of the program by Feb. 1, and these are some of the drugs most likely to make the list.
By Amy Baxter • Jan. 15, 2025 -
FTC releases second report slamming pharmacy benefit managers
Agency commissioners voted unanimously on Tuesday to publish the report, which makes similar allegations against the controversial drug middlemen as the agency’s first report released last summer — but relies on more data.
By Rebecca Pifer • Updated Jan. 14, 2025 -
Merck is broadening its pipeline as Keytruda’s patent cliff looms
Merck looks ahead with an array of cardiometabolic, immunology, neuroscience and ophthalmology pipelines.
By Alexandra Pecci • Jan. 14, 2025 -
Year in Preview
Will 2025 mark pharma’s pivot to bigger deals after years of lackluster M&A?
A series of uncertainties that kept dealmaking to a minimum last year could be reversing as pharma reloads pipelines ahead of the patent cliff explosion.
By Michael Gibney • Jan. 14, 2025 -
The ability to pivot will be ‘key’ as pharma’s tariff threat looms
Companies are looking to new countries like Vietnam for manufacturing needs, but change comes at a cost.
By Kelly Bilodeau • Jan. 13, 2025 -
FDA ‘stuck to its corner’ with AI draft guidance, leaving plenty of questions unanswered
The first draft guidance provides an initial framework for how the agency will assess AI models in drug development, but the industry is still looking for more.
By Amy Baxter • Jan. 13, 2025