Patient
-
Lilly, Novo test direct-to-employer approach that could cut out PBMs and lower costs
The GLP-1 giants are the first to try out this new model, but more companies may follow.
By Kelly Bilodeau • Jan. 14, 2026 -
Opinion // Year in Preview
PharmaVoice’s Crystal Ball: What’s next for AI and drug R&D
Industry execs foresee drug innovation and AI breakthroughs in 2026.
By Meagan Parrish • Jan. 9, 2026 -
 Explore the Trendline➔
Explore the Trendline➔
 Getty Images
Getty Images Trendline
TrendlineArtificial intelligence & machine learning
After years of excited buzz around the potential of artificial intelligence and machine learning, pharma has begun to realize the true implications and potential value of these technologies.
By PharmaVoice staff -
Another genetic testing company? Why a different approach could boost drug predictions and R&D.
Genetic tests could soon play a larger role in clinical trials and predicting adverse events.
By Alexandra Pecci • Dec. 10, 2025 -
FDA conflicts of interest extend beyond adcomm experts
Many speakers at public meetings report conflicts while showing support for new drugs, according to recent analysis.
By Meagan Parrish • Dec. 5, 2025 -
A drugmaker charts a path through pharma’s ethical minefields
As the science behind pharma breakthroughs becomes more complex, bioethicists are helping companies navigate moral quandaries.
By Kelly Bilodeau • Dec. 1, 2025 -
As Madrigal faces MASH rivals, keeping patients is as critical as finding new ones
Madrigal’s drug Rezdiffra has exceeded expectations, and the company plans to stay ahead of incoming competition with a highly targeted patient approach.
By Kelly Bilodeau • Nov. 19, 2025 -
Pharma’s clinical trial diversity push faces a new threat
Skyrocketing healthcare costs could deliver another blow to the industry’s ongoing quest to boost representation in R&D.
By Meagan Parrish • Nov. 7, 2025 -
Why a treatment older than the FDA is getting new regulatory scrutiny
MAHA-aligned patients and providers are pushing back to ensure access to an animal-derived thyroid hormone treatment.
By Kelly Bilodeau • Oct. 20, 2025 -
Pregnant women, long excluded from drug trials, are back in the spotlight
The White House has raised concerns around women’s health, but R&D on pregnant and breastfeeding women is still lacking.
By Meagan Parrish • Oct. 17, 2025 -
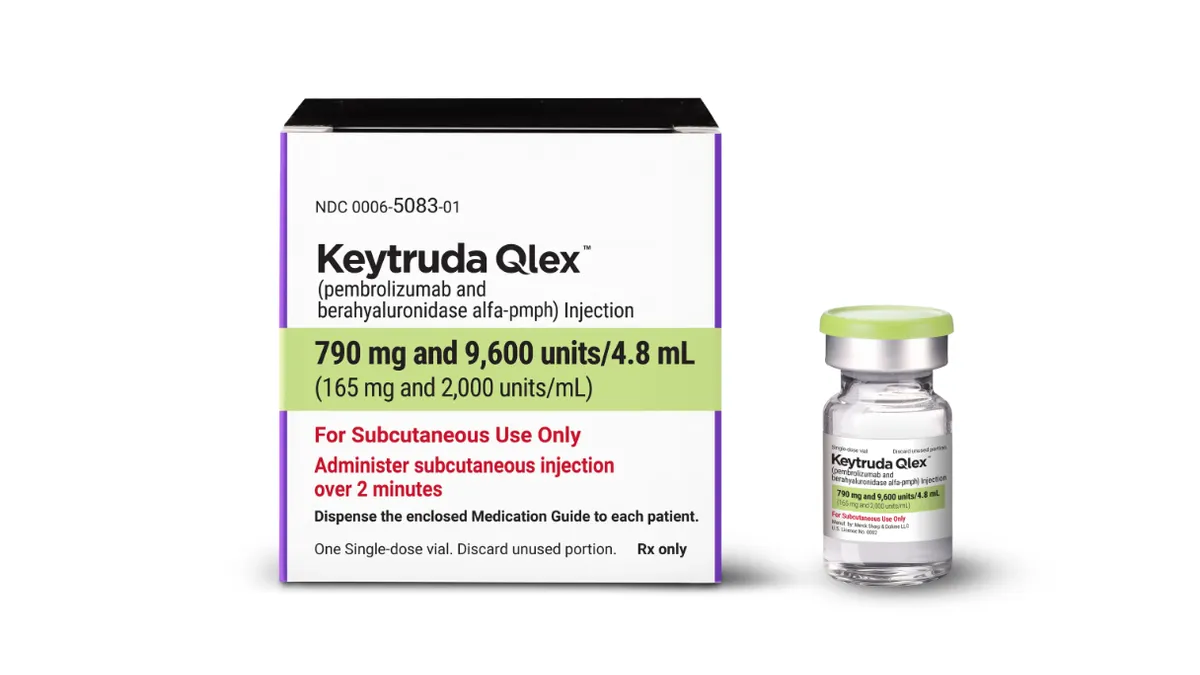
Half of Merck’s sales are in jeopardy. Can Keytruda’s sequel save the day?
This week’s launch of subcutaneously administered Keytruda Qlex gives Merck a safety net for the cancer drug’s daunting patent cliff.
By Michael Gibney • Oct. 2, 2025 -
AstraZeneca’s at-home flu vaccine is a breakthrough that could pave the way for others
FluMist Home could be a test case for at-home vaccines against a backdrop of challenging regulatory shifts.
By Alexandra Pecci • Oct. 1, 2025 -
Top pharma industry conferences in 2026
Where pharma leaders will converge, discuss and navigate the industry’s shifting landscape in 2026.
By Meagan Parrish • Updated Jan. 26, 2026 -
As obesity M&A heats up, pharmas could get ‘blinded by opportunity’
With companies racing to secure GLP-1-related assets, there may be risks for moving too fast.
By Kelly Bilodeau • Sept. 29, 2025 -
From atop Mt. Fuji, a Takeda team heralds a need for human plasma
The climb aimed to raise money and awareness as Takeda ramps up its plasma-derived therapy presence in Japan.
By Alexandra Pecci • Sept. 16, 2025 -
4 years from clinic to approval — Precigen’s ‘unprecedented’ FDA nod and what comes next
The company scored the first-ever approval to treat a potentially deadly infection leveraging its gene therapy platform.
By Meagan Parrish • Sept. 12, 2025 -
Ozempic for cancer? Signs point to potential benefits of GLP-1s in oncology.
Growing research suggests various ways weight loss drugs could have an impact in cancer care.
By Michael Gibney • Sept. 11, 2025 -
Inside pharma’s race to deliver new treatments for dwarfism
By targeting the underlying genetic mutation that drives the condition, researchers hope to increase height and address debilitating complications.
By Kelly Bilodeau • Sept. 8, 2025 -
At Senate hearing, lawmakers express dissatisfaction with RFK Jr.’s vaccine moves
Robert F. Kennedy Jr. faced criticism Thursday even from Republican lawmakers over changes to federal vaccine policy as well as a leadership crisis at the CDC.
By Delilah Alvarado , Jonathan Gardner • Sept. 5, 2025 -
Why an FDA decision for a new drug could ripple through the ultra-rare disease space
All eyes are on Stealth BioTherapeutics as it awaits a long-delayed approval for its first-in-class treatment for Barth syndrome.
By Alexandra Pecci • Aug. 26, 2025 -
As gene therapies falter on the market, this biopharma is aiming to defy the odds
After a strong launch for its cancer gene therapy, Ferring Pharmaceuticals is setting the stage for long-term growth.
By Meagan Parrish • Aug. 8, 2025 -
Novartis’ Zolgensma sales fall again as gene therapy market woes persist
Novartis has seen sales decline in a difficult gene therapy market. Is there hope on the horizon?
By Michael Gibney • July 22, 2025 -
Pricing watchdog will take on the rising cost of drugs at launch
ICER’s upcoming report could shed light on how the launch price of newly approved drugs affects overall cost and access from a patient perspective.
By Michael Gibney • July 10, 2025 -
GLP-1 roadblocks: Discontinuation rates soar as insurers clamp down
As more drugmakers aim for the lucrative weight loss market, patient challenges linger.
By Amy Baxter • July 7, 2025 -
First 90 Days
A biopharma bolsters patient centricity and trial diversity goals through newly created role
Ardelyx’s newly minted chief patient officer is infusing patient experiences into every element of the business.
By Alexandra Pecci • July 1, 2025 -
Biogen scores Cannes Festival win with dark humored campaign
The company’s unique campaign for a rare, terminal illness nabbed millions of views and a spot on the main stage at the Cannes Lions Festival of Creativity.
By Alexandra Pecci • June 24, 2025