Biotech: Page 11
-
Private biotech M&A surges amid difficult IPO market
Private biotech company acquisitions are on their fastest pace in years, a trend some in the industry say is driven by the abundance of mature, but not yet public, drug startups.
By Gwendolyn Wu • July 23, 2024 -
Q&A
Bird flu puts BARDA back in the spotlight
BARDA director Gary Disbrow explains the agency’s focus, how companies can get their “foot in the door” and why platforms are so important for preparedness.
By Alexandra Pecci • July 23, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Getty Images
Getty Images Trendline
TrendlineOncology R&D
Cancer research remains a prime focus for the industry and often leads to pharma’s most impactful breakthroughs.
By PharmaVoice staff -
Kyverna follows oncology’s CAR-T cell playbook for an ultra-rare autoimmune disorder
The pharma company is tackling the “stiff person syndrome” that afflicts Celine Dion in a phase 2 trial for its lead CAR-T candidate.
By Amy Baxter • July 19, 2024 -
Q&A
Amylyx’s CEOs on their ALS setback and quest to revamp the pipeline
Co-CEOs Josh Cohen and Justin Klee reflect on what they learned from Relyvrio’s failure and how they’re carrying those lessons forward with other drugs.
By Meagan Parrish • July 17, 2024 -
Biotech Spotlight
A new biotech tackling IBD joins a race against Lilly and Gilead for a promising pill
Ensho Therapeutics just launched but is already generating buzz with its phase 2-ready clinical program for inflammatory bowel disease.
By Alexandra Pecci • July 17, 2024 -
Has pharma emerged from the ‘age of uncertainty’?
An industry reeling from a precarious market is showing signs of stabilizing as the future becomes a little less cloudy, an Evaluate report suggests.
By Michael Gibney • July 16, 2024 -
Is a better CAR-T cell therapy option on the horizon?
In vivo CAR-T cell technology has the potential to solve some major issues in the field and could enter the clinic this year.
By Kelly Bilodeau • July 15, 2024 -
Biotech Spotlight
Mining clues from a library of brain tissue samples, Cerevance takes a precision approach to CNS disorders
Mid-stage data from the company’s lead candidate in Parkinson’s is expected later this year and could tee up an IPO.
By Meagan Parrish • July 15, 2024 -
Could pharma’s blockbuster immunotherapies work in dogs?
Vetigenics believes it’s found a way to make pricey antibody-based technologies more accessible for animals.
By Alexandra Pecci • July 9, 2024 -
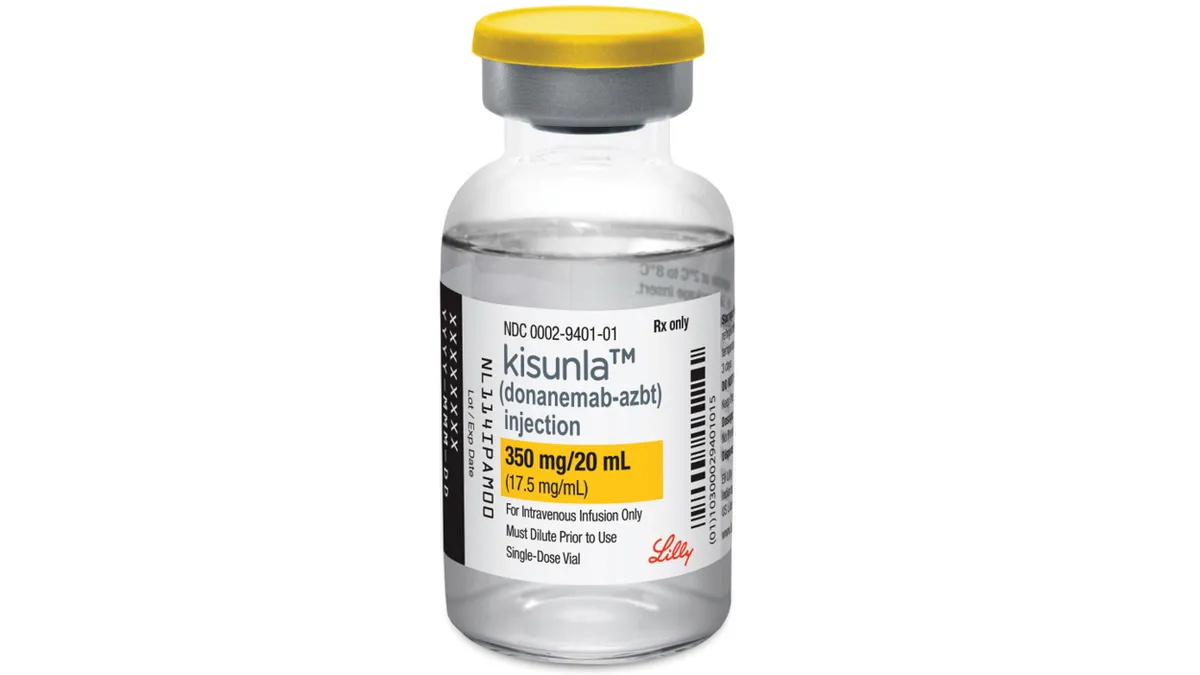
FDA approval is just the beginning. Now Lilly’s Kisunla faces the tough Alzheimer’s market.
Recent history suggests an Alzheimer’s approval doesn’t necessarily translate to market triumph. Perhaps for Eli Lilly’s Kisunla, this time could be different.
By Michael Gibney • July 9, 2024 -
In the rapidly shrinking COVID market, this company still sees opportunity
GeoVax is developing a next-gen COVID vaccine targeting a population that’s so far been elusive: immunocompromised patients.
By Alexandra Pecci • July 3, 2024 -
Q&A
How BeiGene is taking on established blockbusters with an expanding blood cancer arsenal
BeiGene’s Brukinsa is the first in a hematology franchise that the company hopes will rival top-sellers and introduce a new lineup of medicines for patients.
By Michael Gibney • July 2, 2024 -
Biotech Spotlight
Protein power: a biotech mining viruses to fight disease
Flagship Pioneering-backed Prologue Medicine is harnessing the evolutionary tactics of viral proteins to systematically find new drugs.
By Kelly Bilodeau • July 1, 2024 -
Up-and-comers look for an edge in the bustling ADC field
The next wave of targeted ADC therapies is on the horizon, and these three potential rising stars could help carry the field forward.
By Meagan Parrish • June 28, 2024 -
Women with a common hormonal disorder have few good treatment options. Could GLP-1 drugs help?
Obesity drugs like Wegovy are proving useful in many other diseases. Polycystic ovary syndrome, a chronic condition that can cause infertility, may be one.
By Delilah Alvarado • June 27, 2024 -
Don’t call it a comeback: Biotech’s CNS resurgence forges Big Pharma connections
Neuroscience pipelines are reawakening as biotechs prove to the pharma giants that the field is still worth the investment.
By Michael Gibney • June 27, 2024 -
After 33 years, Geron’s first approval marks a turn in Nobel-winning science
The decades-long story of how a first-in-class blood cancer treatment went from bench to bedside.
By Alexandra Pecci • June 26, 2024 -
Ahead of its upcoming decision date, a competitor takes aim at KarXT
The FDA isn’t slated to render a decision about BMS and Karuna’s potential breakthrough schizophrenia med until September — but a biotech is already hoping to be hot on its heels.
By Kelly Bilodeau • June 25, 2024 -
Q&A
Can Moderna’s COVID-flu combo shot improve flagging vaccination rates?
A COVID and flu duo could ease manufacturing and administration, potentially driving higher vaccination rates, said Moderna’s VP of North American medical affairs.
By Michael Gibney • June 25, 2024 -
Q&A // First 90 Days
After an obesity deal with Novo, Omega’s new business head is on the hunt for more partners
With a career focused on striking deals, Kaan Certel is hoping to lead the Flagship-backed biotech with an mRNA platform into its next phase of growth.
By Amy Baxter • June 24, 2024 -
3 windows of opportunity in women’s health
Despite a recent surge of interest, the femtech and women’s health markets are far from reaching their full potential.
By Alexandra Pecci • June 21, 2024 -
New data showcase promise, growing pains of CAR-T in autoimmune disease
One expert described trial results presented at EULAR last week as “unprecedented.” But reports of relapses in some patients drew questions about the therapies’ ultimate potential.
By Ben Fidler • June 20, 2024 -
Avoiding the pharma ‘junk pile’ with launch readiness, pipeline rigor and ‘quick kills’
Drug launches have underperformed expectations at a high rate, and pharmas need to get better at thinning the pipeline to make room for the real wins, says a life sciences consultant.
By Michael Gibney • June 18, 2024 -
Making Moves
Regenxbio CEO to step down after 15 years
Kenneth Mills will become chair of the gene therapy developer's board while Curran Simpson, the current chief operating officer, will take his place as company head.
By Ned Pagliarulo • June 13, 2024 -
Lilly’s adcomm win shows amyloid and tau will likely dominate Alzheimer’s — for now
Lilly is the latest to approach the coveted Alzheimer’s approval, but a slate of drugmakers are lining up clinical trials in amyloid and tau to take a shot at improving patient outcomes.
By Michael Gibney • June 13, 2024