Seasonal vaccination rates are in a dire state — although the COVID-19 pandemic highlighted shortcomings in access and public trust, those lessons haven’t translated into more shots in arms. But vaccine makers and healthcare experts hope new technologies will improve infectious disease prevention as the situation worsens.
In the 2023-24 respiratory season, the number of U.S. children who died from the flu reached 199, according to a CDC report, and more than 80% of those deaths occurred in children who weren’t fully vaccinated. As more data came in following the initial release, one more death was reported, making it the most deadly flu season for U.S. children on record.
And yet, vaccination rates are on the decline. Children aged 2 and younger born at the height of the pandemic had lower vaccination rates across all diseases than those born a year earlier. In flu alone, about 56% of children born in 2020 and 2021 were fully vaccinated — down from 63% for those born in 2018 and 2019.
Less than half of U.S. adults were vaccinated against the flu last year, and public hesitation has risen to a new level, according to the National Foundation for Infectious Diseases. Only one in five adults expressed concerns about respiratory diseases like flu, COVID-19, RSV or pneumococcal disease.
And CDC projections estimate the 2024-25 respiratory disease season could look like last year’s in terms of hospitalization burden.
As public health messaging falls short of improving uptake, vaccine makers and other healthcare companies are embarking on a mission to make infectious disease prevention more feasible by deploying different technologies.
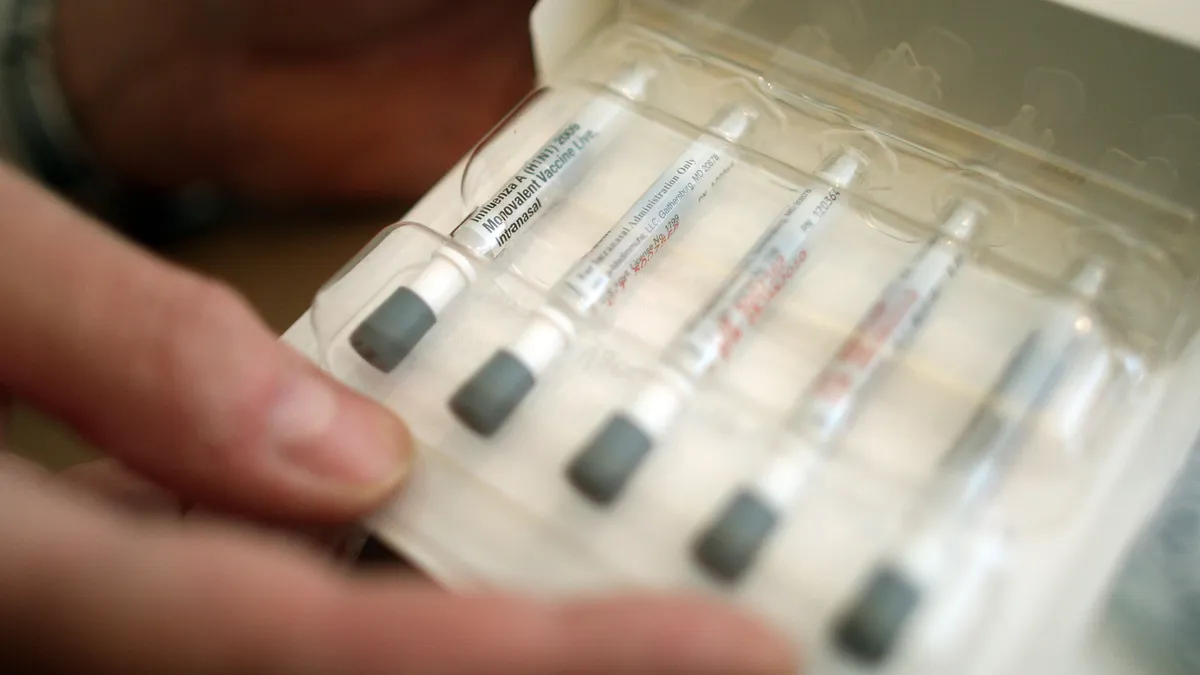
Nasal options
FluMist, AstraZeneca’s nasal flu vaccine, has been available since 2003 with more than 200 million doses administered globally in that time. But the FDA recently approved the spray for at-home use, giving people the option to skip the trip to the pharmacy.
Although at-home delivery of FluMist won’t be available until the 2025-26 respiratory disease season, the FDA OK could help AstraZeneca gain a better foothold in the market.
In the 2022-23 season, FluMist accounted for just over 2% of flu vaccines for U.S. children aged 2 to 4, less than 14% of children aged 5 to 17, and less than 4% of adults under the age of 49, according to an analysis by GlobalData. But at-home delivery could change that next year.
“By increasing access to FluMist, there is potential for these percentages to increase,” said Stephanie Kurdach, infectious disease analyst at GlobalData, in a statement. “It is possible that we will see an increase in U.S. patient shares of FluMist, given the convenient route of administration and ease of access, as well as an overall increase in seasonal influenza vaccine uptake.”
A nasal option might also be on the way for COVID-19 — an NIH-sponsored early-stage trial of an spray began enrolling patients this summer to mark first-in-human use of the vaccine developed by National Institute of Allergy and Infectious Diseases researchers. The program is part of BARDA’s Project NextGen, which coordinates public and private developers of new vaccine technology.
Nasal options could also be more effective, according to Jacco Boon, a professor and molecular microbiology, pathology and immunology at Washington University School of Medicine in St. Louis, where he is leading the development of another nasal vaccine for COVID-19.
“This study shows that mucosal vaccines are superior to injected vaccines in terms of limiting viral replication in the upper airways and preventing spread to the next individual,” Boon said in a statement. “In an epidemic or pandemic situation, this is the kind of vaccine you’re going to want.”
An AI approach
Misinformation and “deliberate ignorance” has driven much of the anti-vaccination sentiment that arose during the pandemic, according to a study from researchers at the Max Planck Institute for Human Development. No solution will work to get everyone on board with vaccination, but for those on the edge, some AI developers have begun tackling the problem.
A 2021 study from France’s National Centre for Scientific Research found that an AI chatbot designed to answer questions about COVID-19 vaccines was able to increase participants’ willingness to get vaccinated.
After just a few minutes with the program, 37% exited with more positive views of vaccination and 20% were more open to receiving one. In a control group that received an informational flier, changes were negligible, according to the study.
By identifying misinformation around side effects, for instance, which has been one of the main drivers of vaccine hesitancy, AI could play a role in presenting data in a way that is tailored to a person’s inherent willingness, other researchers have suggested.
Getting to the appointment
Another driver of vaccine hesitancy is the inconvenience of making it to the pharmacy, and sometimes, a reminder is necessary.
New research from health software company Phreesia showed that patients who received messaging related to vaccines ahead of an appointment were more than twice as likely to receive incremental vaccinations. Almost two-thirds of the patients said the messaging made them more likely to discuss vaccines with their doctor.
“Phreesia research has shown time and again that the point of care is an important place to engage patients while they’re making decisions about their health,” said Jai Seth, associate director of strategic research at Phreesia. “We look forward to continuing to study the impact that outreach in this setting can have on vaccine attitudes and behaviors and exploring new ways our platform can be used to support vaccination in this critical moment.”