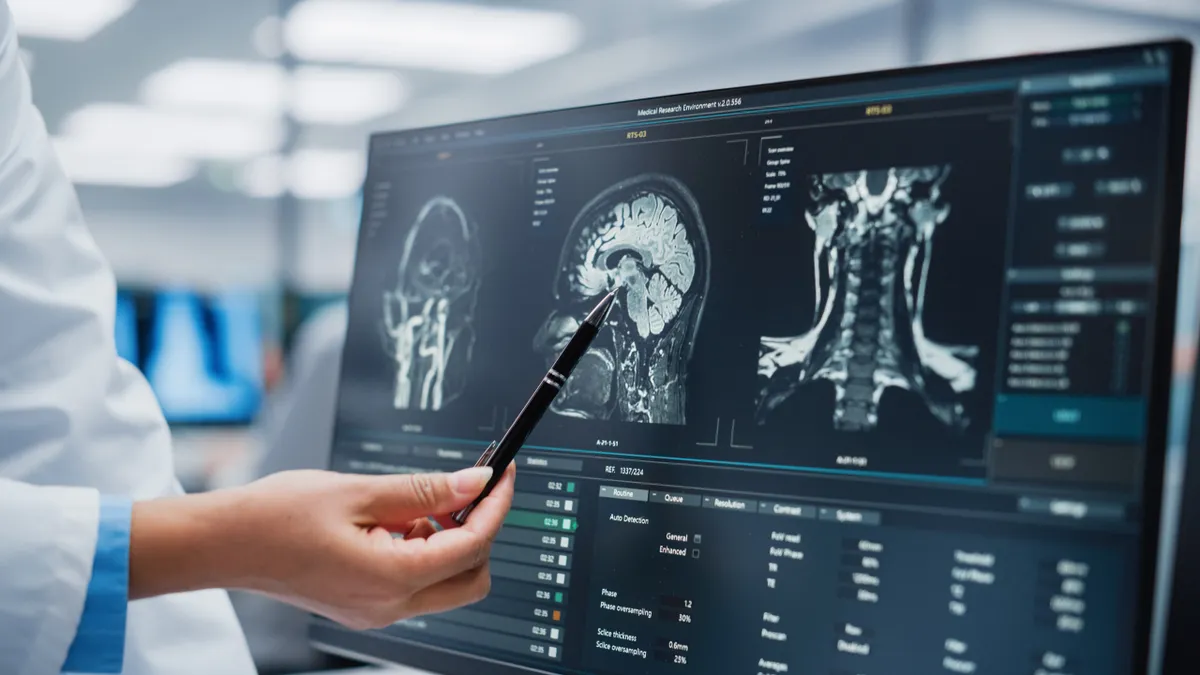
Researchers in any field know that careers are forged not necessarily from once-in-a-lifetime "eureka" moments but from a series of iterative observations that add to a body of work — discipline, patience and perseverance are the key to scientific discovery.

For Sindhu Ramchandren, director and clinical leader of neuroscience at Janssen, keeping that perspective is especially important in developing drugs for rare diseases, where a community of patients await breakthroughs that take time and commitment from a core group of devoted minds.
"I think the eureka moments come, but they are few and far between. It's very easy to get emotional because you see the community and it's very easy to hope for the best," Ramchandren said. "But as a scientist, those moments where you say, 'I'm seeing a signal,' can only come at the end when you're looking at the vast majority of data altogether."
Ramchandren turned a fascination with neurology and how specific parts of the brain impact certain parts of the body into a career in the rare neuromuscular space. Patients with diseases like muscular dystrophy, myasthenia gravis and chronic inflammatory demyelinating polyneuropathy (CIDP) have been her specialty since her days in academia at the University of Michigan and as director of a clinic for rare neurology.
"I always was very privileged to take care of these individuals with a lot of life lessons learned from them — how they handle diversity and how they maintain hope that there is something coming in the future that will improve if not for them, than others with this disease," Ramchandren said. "That's something I always carry with me."
And today, she carries those lessons into the drug development arena, leading clinical programs to treat neurological diseases like myasthenia gravis with a therapy called nipocalimab, which is designed to block receptors that lead to the miscommunication between nerves and muscles.
Harnessing the immune system
Janssen showed in a mid-stage clinical trial that nipocalimab significantly improved symptoms in patients with myasthenia gravis, which is associated with trouble walking, swallowing, breathing and sight. This is a result of autoantibodies that form in the spaces where the brain speaks to the muscles, causing the patient's own immune system to disrupt those signals.
"The body starts recognizing itself as foreign — in myasthenia gravis, what happens is that the immune system forms autoantibodies that target muscle proteins that are responsible for communicating with the nerves," Ramchandren explained.
"I think the eureka moments come, but they are few and far between. It's very easy to get emotional because you see the community and it's very easy to hope for the best."

Sindhu Ramchandren
Director and clinical leader, neuroscience, Janssen
The standard of care for a disease like myasthenia gravis or CIDP is currently broad immunosuppression, which comes with many side effects. Drugs like steroids or those used in chemotherapy can also take a long time to start working. And suppressing the immune system can leave a patient vulnerable to infections and other risks.
"About 20% of patients with myasthenia gravis don't respond to the current standard of care, so there's an unmet need where we need something that's more specific and faster," Ramchandren said.
Nipocalimab, on the other hand, is not an immunosuppressant but rather an immunomodulator. Thus, patients who cannot tolerate a weakening of the immune system — elderly, pediatric or pregnant patients, for instance — might have a better chance with nipocalimab.
For Janssen, which has a deep pipeline in neuroscience, nipocalimab represents not just a chance to treat patients with one or two rare diseases, but potentially a wide range of diseases including hemolytic diseases of fetuses and newborns.
"The specific opportunity that Janssen saw with nipocalimab was that it could be a very unique product to address multiple rare diseases simultaneously," Ramchandren said. "Pharmaceutical companies have many options out there for them to say, 'What should we go after?' and I think the unmet need in rare neurology and the fact that this molecule could address so many different diseases was very attractive."
Rare disease roadblocks
Challenges in addressing rare diseases are numerous, and one of the greatest hurdles is in diagnosing and finding patients. Not every disease has a biomarker that can be pinpointed. In patients with myasthenia gravis, there is often an autoantibody present, but not in all patients, Ramchandren said. What's more, a general physician does not always know to look for the rarer indications of the disease. Thus, it can take years for a correct diagnosis.
Beyond these challenges, Ramchandren said that development considerations for rare disease drugs, such as a disconnect between a trial design and what patients need, are so complicated they can limit investment.
"There's not a very good algorithm to take those calculations into account," Ramchandren said. "So there needs to be a larger discussion beyond showing what is effective in a clinical trial to looking at the endpoints that the rare disease community really wants us to be looking at, and how to show the value to the larger group of stakeholders to bring these drugs into the market."
In studying nipocalimab for myasthenia gravis, Janssen explored not only the levels of autoantibodies but also a measure of daily living that indicates symptom severity. Both improved in the phase 2 study.
The pharma giant is enrolling a late-stage study in adults with myasthenia gravis. Two ongoing mid-stage nipocalimab studies include those in pediatric patients with myasthenia gravis and adult patients with CIDP.
"As a physician, this is such an exciting time for autoantibody diseases that we have these new mechanisms," Ramchandren said. "We're really excited and hopeful about the future of public health."