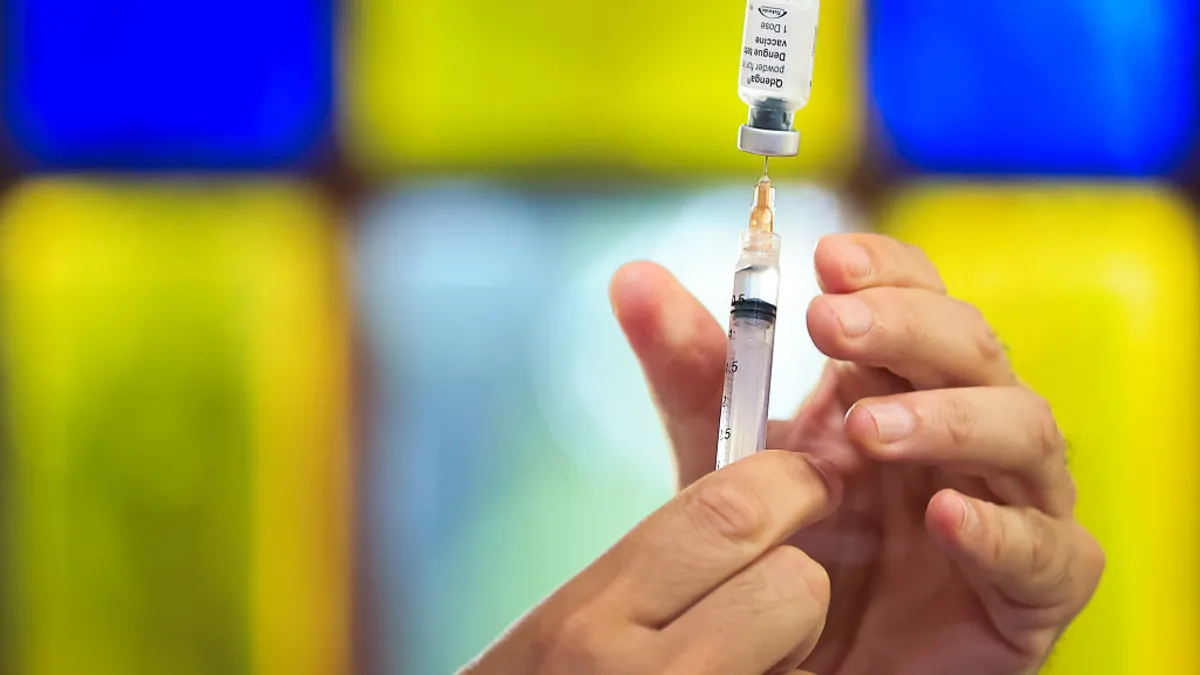
Derek Wallace was in a dengue-focused ICU in Thailand in 2009 when he first saw the devastating, up-close impact of the disease, also known as the painful “breakbone fever.” Facing a shortage of space, children shared beds while parents slept together on mattresses tucked underneath.
“That’s been the case for decades in some countries,” said Wallace, who was a clinical team leader for Sanofi's dengue vaccine studies at the time and is now president of Takeda Pharmaceutical’s global vaccine business unit. “But what’s really important is that it’s getting worse. There’s 30 times more dengue than there was 50 years ago, and there’s eight times more dengue now than there was 20 years ago.”
The mosquito-transmitted virus is among the top 10 global health threats designated by the World Health Organization. There were a record 6.43 million cases of dengue and nearly 7,000 deaths worldwide in 2023 — the largest outbreak ever recorded. Dengue is also spreading faster in places where it hasn’t before thanks to factors like climate change, urbanization and increased global travel, according to the WHO.
“Dengue is moving, and we are starting to get a ton of locally acquired dengue fever cases in the U.S., specifically in Puerto Rico, Virgin Islands and Florida,” said Katelyn Jetelina, an epidemiologist and data scientist known for a popular newsletter. “And we expect this to continue increasing over time.”
Despite the rapid global spread, there is no specific treatment for dengue, and worldwide vaccine availability is limited.
Sanofi’s Dengvaxia is currently the only FDA-approved dengue vaccine. But the company is discontinuing the shot in the third quarter of 2026 “due to low global demand for this product,” a Sanofi spokesperson said via email.
Takeda’s two-dose dengue vaccine, Qdenga, is approved in 40 countries excluding the U.S, and Takeda withdrew its U.S. application in 2023.
“Some additional data was requested that we couldn't provide,” Wallace said, but declined to provide details.
Although the U.S. IND is still open for Qdenga, “there are no specific updates to share at this time about the timing of supplying additional data or resubmission,” a Takeda spokesperson said via email.
The vaccination challenge
Contracting most viral diseases for the first time will arm you with antibodies that fend off subsequent infections. But that’s not the case with dengue, thanks to antibody dependent enhancement that stems from dengue’s four closely related but distinctly different viral serotypes.
“It’s challenging because it's a very unique disease in which secondary infections are more severe than the first infections,” said Jetelina.
Sanofi’s vaccine has long been mired in controversy for this reason. Receiving Dengvaxia could act as a first dengue infection, resulting in a more severe illness after contracting the virus a second time. As a result, Dengvaxia can only be administered to people who’ve had dengue before, and people must be screened for a previous infection before getting the shot.
“Dengue is spreading to areas where it was not prevalent, and it’s affecting more susceptible populations that have no immunity whatsoever."

André Siqueira
Head, Dengue Global Program, Drugs for Neglected Diseases
But that’s not how Dengvaxia’s initial Philippines rollout went — a mass vaccination program didn’t take this screening step, leading to more severe disease among some patients and potentially some deaths.
The onerous double whammy of needing a pre-vaccine screening combined with a three-dose regimen — not to mention eroded public trust in vaccines — has meant that demand for Dengvaxia is so low, Sanofi is throwing in the towel.
“While we have made consistent efforts to facilitate its access and the implementation of the ‘Screen & Vaccinate’ approach as recommended by WHO in 2018, uptake and demand has remained low,” the Sanofi spokesperson said. “Despite an increased prevalence of dengue in different parts of the world, there’s only one Dengvaxia immunization program in place due to the complexity of implementing this product.”
Qdenga doesn’t have these limitations. According to Takeda, the two-dose jab is “the only vaccine approved for use regardless of previous dengue exposure.” The WHO doesn’t recommend pre-vaccination screenings for Qdenga administration in settings with high dengue transmission, although local approvals may vary. Qdenga has been shown to be 61% effective against symptomatic disease and 84% effective in preventing hospitalization. Still, it’s not approved in the U. S.
Other vaccines are in development, including the single-dose Butantan–Dengue vaccine that showed positive phase 3 results.
Dengue’s spread
These vaccination and treatment challenges only make dengue’s spread more worrisome.
“Dengue is spreading to areas where it was not prevalent, and it’s affecting more susceptible populations that have no immunity whatsoever,” said André Siqueira, head of the Dengue Global Program at the nonprofit Drugs for Neglected Diseases.
He points to southern Brazil, where dengue’s recent and rapid spread has overwhelmed health systems in an area that previously didn’t have any infections.
“What has happened the last few years in Brazil may mirror what can happen in these places where dengue transmission is still picking up,” Siqueira said.
The U. S. is one of those places. In Puerto Rico, a record-high dengue surge sparked a public health emergency in March, and in the Florida Keys, public health officials issued an alert last summer.
Dengue thrives in warm urban areas, and the mosquitoes responsible for its spread can efficiently reproduce even in small pockets of water. Therefore, a warming climate with heavier rainfalls, along with increased urbanization, are all fueling the spread. Moreover, these mosquitoes have evolved resistance to insecticides and are adapting to different conditions, including more temperate climates, Siqueira said.
That’s why public health organizations are taking action. DNDi kick-started its Dengue Alliance in 2022; the WHO launched a global strategic plan against dengue earlier this month; and Takeda partnered with the Indian pharma Biological E. Limited earlier this year to accelerate access to the vaccine in endemic areas.
But public health officials and local health systems are still racing against the clock, calling for more vaccines, dengue-specific treatments and improved diagnostics.
“There is a perception that we’ll find one solution for dengue,” Siqueira said. “What we probably need in the most affected areas is an integrated approach.”