What is “Digital Clinical?" As Morpheus tells Neo in the film The Matrix, “No one can be told what the Matrix is. You have to see it for yourself." Today we sometimes find ourselves immersed in our own real-life version of the Matrix, as our personal data accumulates on computer servers somewhere in the mysterious “cloud," creating the digital equivalent of our fingerprint, or what we call, our unique Code Halo. Our Code Halos permeate our lives — our work, our families, and our friends — as we have learned everything can be digitized: words, voices, and images, all converted into a series of zeroes and ones. Almost every electronic transaction we conduct — from making credit card purchases to downloading articles, what we watch on television or post on Facebook, what we thought were private e-mail discussions, data from our wearable devices, or what we let organizations know about our personal health information, even the data generated by our household appliances — all of these form our individual Code Halos.
What is “Digital Clinical?" As Morpheus tells Neo in the film The Matrix, “No one can be told what the Matrix is. You have to see it for yourself." Today we sometimes find ourselves immersed in our own real-life version of the Matrix, as our personal data accumulates on computer servers somewhere in the mysterious “cloud," creating the digital equivalent of our fingerprint, or what we call, our unique Code Halo. Our Code Halos permeate our lives — our work, our families, and our friends — as we have learned everything can be digitized: words, voices, and images, all converted into a series of zeroes and ones. Almost every electronic transaction we conduct — from making credit card purchases to downloading articles, what we watch on television or post on Facebook, what we thought were private e-mail discussions, data from our wearable devices, or what we let organizations know about our personal health information, even the data generated by our household appliances — all of these form our individual Code Halos.
Digital Clinical
The digitization of our lives began with the personal computer, and then it accelerated with the emergence of the Internet and the rapid uptake of mobile devices. It took just a few years to appreciate the enormity and power of the vast stores of digital information, or big data.
However, the life-sciences and healthcare industries were reluctant to embark on the transformative activities made possible by the rise of these technologies and the Code Halos they enable — what is now the backbone of what we call Digital Clinical. The hesitation to act on digitization and data stores had validity, particularly because of concerns relating to personal health information (PHI) and regulations such as HIPAA and EU Data Privacy acts. Yet while privacy and security issues must be addressed, the vast amounts of data available in Code Halos create unlimited potential benefits for Digital Clinical and reshaping life sciences industry processes.
Current State
 Today, we remain impressed, but no longer awed by available technology that helps diagnose, monitor, and treat patients. Nor do we blink an eye that our medical records (like our credit card data) are floating in a secure cloud and can be accessed almost anywhere by our doctors to inform treatment decisions. The life-sciences arena now understands that these enabling digital technologies are critical to improving clinical trial operational productivity. Johnson & Johnson Chairman Joaquin Duato has stated that J&J is looking to collaborate and create value with a broad expanse of technology companies to find solutions that use big data, sensors and computing to accelerate the development of new drugs, biologics and devices, and deliver better outcomes to patients.
Today, we remain impressed, but no longer awed by available technology that helps diagnose, monitor, and treat patients. Nor do we blink an eye that our medical records (like our credit card data) are floating in a secure cloud and can be accessed almost anywhere by our doctors to inform treatment decisions. The life-sciences arena now understands that these enabling digital technologies are critical to improving clinical trial operational productivity. Johnson & Johnson Chairman Joaquin Duato has stated that J&J is looking to collaborate and create value with a broad expanse of technology companies to find solutions that use big data, sensors and computing to accelerate the development of new drugs, biologics and devices, and deliver better outcomes to patients.
In fact, an overwhelming majority of life-sciences leaders believe that mobile technologies, along with data mining and analytics, have the greatest likelihood to deliver near-term strategic benefits. Nonetheless, these leaders are “increasingly anxious about the speed at which technology is evolving" but are “pressing ahead with the digital transformation of their companies" and “reaping significant benefits in terms of digital trust, operational efficiencies and the ability to innovate."
Digital Clinical — Investigators and Patients
So, what are the new digital technology approaches and data analytics capabilities that industry academia, government regulators, payers, investigators, and patients expect to improve the clinical trial paradigm? First, organizations such as TransCelerate Biopharma and the Society for Clinical Research Sites are leading the renewed emphasis to engage more productively with investigators and patients to design trials that satisfy global regulatory requirements, as well as meet payer value and outcome expectations while reducing administrative burden.
TransCelerate is leveraging technology — coupled with industry-accepted precompetitive training and credentialing, as well as by deploying standard processes, tools, and portal interfaces — to provide already overburdened investigator teams with more time to focus on patient care and other value-add activities. However, a 2014 follow-up study by Kenneth Getz of the Tufts Center for the Study of Drug Development surprisingly found that our words and our deeds are sometimes discordant. The study noted a nearly 6% rise in “Phase III protocol procedures [that] supported non-core endpoints and objectives," thereby adding to (not reducing) the investigator — and patient — burden.
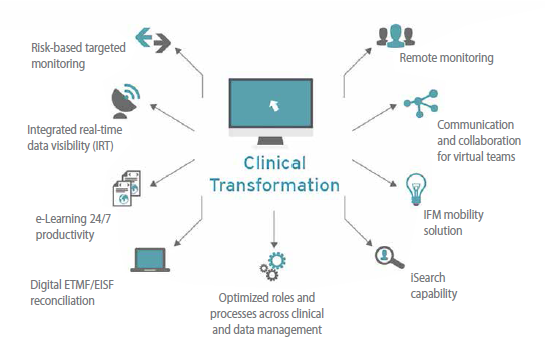 More attention also is being paid to patients; call it patient-centricity or patient engagement or, perhaps, just common sense. From the product development standpoint, patient recruitment and retention remain the top two clinical trial processes severely in need of improvement and optimization; this makes it an imperative to find ways to engage with patients more effectively. In response, clinical trialists are investing more upfront time designing patient-friendlier studies, collecting information from any number of digitized sources, and using technologies to support patients and keep them interested and compliant, respectively. Likewise, online Digital Clinical patient recruitment approaches — such as social media, advertising, patient advocacy (via blogs and podcasts) and more precise EHR data mining techniques — are being adopted that complement, but do not supplant, conventional media and direct mail outreach. Though each of these avenues is not fully proven, favorable evidence is mounting.
More attention also is being paid to patients; call it patient-centricity or patient engagement or, perhaps, just common sense. From the product development standpoint, patient recruitment and retention remain the top two clinical trial processes severely in need of improvement and optimization; this makes it an imperative to find ways to engage with patients more effectively. In response, clinical trialists are investing more upfront time designing patient-friendlier studies, collecting information from any number of digitized sources, and using technologies to support patients and keep them interested and compliant, respectively. Likewise, online Digital Clinical patient recruitment approaches — such as social media, advertising, patient advocacy (via blogs and podcasts) and more precise EHR data mining techniques — are being adopted that complement, but do not supplant, conventional media and direct mail outreach. Though each of these avenues is not fully proven, favorable evidence is mounting.
Clearly, all stakeholders have skin in the clinical trial optimization game, even health authorities, as evidenced by the FDA’s “Voice of the Patient" program, which aims to “systematically gather patients’ perspectives on their condition and available therapies to treat their condition."
Notwithstanding these earnest patient engagement efforts, Marc Boutin, JD, of the National Health Council, writes in the American Journal of Managed Care that “efforts to advance the concept are fragmented and disjointed." Nonetheless, he argues that the industry is committed to assessing “different engagement strategies [in order to] better understand the distinct perspectives of patients [and] incorporate these data into the decision-making process for new treatments."
mHealth
Another ongoing Digital Clinical revolution is the rapidly accelerating adoption of mobile, self-serve diagnostic, monitoring, and treatment modalities (mHealth) in both the healthcare and clinical trial arenas. Mobile technology already is having a material impact on the current and future delivery of healthcare; trends show that smart mobile tools enable greater individual accountability for health management, and potential reductions in medical spending, as individuals self-monitor conditions more effectively. As for clinical development, mobile digital technology provides the dual benefit of reducing investigator and patient burden (e.g., decreasing the frequency and/or the duration of clinic visits), even if the industry continues to increase the number of data points collected, by capturing timely and higher quality data remotely, using at-home, wearable, implantable (even ingestible) devices.
Moreover, the industry should applaud the sensible approach the FDA has taken regarding the regulation of mobile applications, as it has chosen to narrow its oversight to focus on mobile medical apps that present a greater risk to patients if they don’t work as expected.
Taken a bit further, digital technology makes it possible to continuously monitor, in real-time, the well-being of patients during and after clinical trials to collect data and insights into patient health and compliance, as well as the safety and effectiveness of the therapy. Digital tools enable the swift, secure collection of large volumes of accurate, consistent data on which analysis can then be quickly performed, such as comparing therapies and assessing efficacy. These data then add a clinical research component to each person’s Code Halo that may then be parsed for further insights into therapy performance.
Making Sense of it All
As the data volumes in our Code Halos expand, so do clinical trial databases; therefore, we need to be able to intelligently harness and make sense of the data in order to make informed, timely, actionable decisions. However, the insights we seek are too often difficult to find. To overcome this challenge, both structured and unstructured data will need to be consumed and surfaced by a team of well-equipped data analysts, who can combine their expertise with digital and mobile analytics technologies to sift through the data to make trustworthy predictions, identify trends, detect safety signals, etc. In fact, McKinsey estimates that applying big data strategies to better inform decision-making could generate up to $100 billion in value across the U.S. healthcare system, in part by improving the efficiency of research and clinical trials.
What’s Next?
Upon meeting Trinity in The Matrix, Neo asks her, “What is the Matrix?" Trinity replies, “The answer is out there, Neo, and it’s looking for you, and it will find you if you want it to." Like Neo, life-sciences companies ‘willing to look for and find it’ are witnessing a wholesale transformation of the clinical trial operational delivery model driven by ever improving digital clinical technology tools. Correspondingly, these tools are expected to improve trial design, yield higher quality data at lower cost and, ideally, accelerate product development cycle times, permitting the faster release of new products to help patients.(PV)
Cognizant is helping life-sciences companies run better by realizing new levels of performance and efficiency, as well as run different by harnessing the power of new digital business capabilities to enrich the sponsor/CRO, the investigator and the patient experience to drive better health outcomes.
For more information, visit cognizant.com/life-sciences