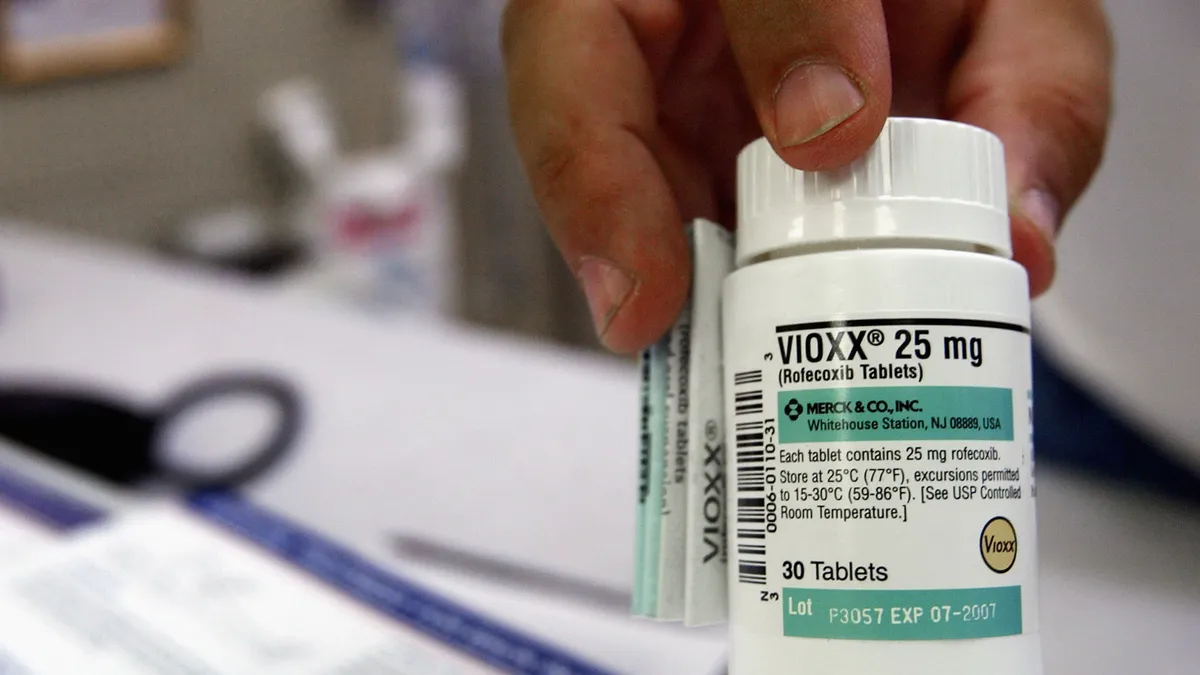
For years, the dramatic saga of Vioxx has been held up as an example of how a pharma company could leverage questionable science to hide the dangers of its drug. After being approved by the FDA in 1999, Vioxx, a Cox-2 selective non-aspirin nonsteroidal anti-inflammatory drug (NSAID) that became a blockbuster for Merck, eventually plummeted from grace amid allegations that it led to thousands of deaths.
Ultimately, Merck & Co. paid $4.85 billion dollars in 2007 to settle a large number of patient lawsuits related to the painkiller. The drugmaker had already pulled Vioxx off the market in 2004 after studies revealed it could double the risks of heart attacks and strokes.
But although Vioxx came to be viewed as something of a pariah, Bruce Register, founder and CEO of BriOri BioTech says he thinks the drug’s bad rap is unfair. He was working at Merck as a medical science liaison for Vioxx, managing the south-central U.S. when the drug was abruptly pulled off the market, leaving patients stunned.
“I would have patients approach me and say, ‘You can't take it off the market, it’s the only thing that touched my pain, you’ve really got to leave it on the market,’” Register says. “The physicians hoarded everything that they had, they would not give back the samples to the sales reps or to Merck.”
Now, Register believes, the time is right for Vioxx to stage its comeback.
Based in California, BriOri BioTech, a preclinical company, says it’s developing a pipeline of topical pain relievers, among which is Relÿva. Designed to harness Vioxx’s potent pain-reducing power for conditions like arthritis, the cream comes with an improved safety profile. With the global market for topical pain-relievers slated to grow in the coming years, and the industry focused on non-opioid options for chronic pain conditions, Register says the product could fill a major unmet need.
It’s one of several ways Vioxx has been vindicated in recent years. After Merck pulled it off the market, an FDA investigation revealed the increased cardiovascular risk was seen in all NSAIDs — not just Vioxx. In 2005, the FDA required a black box warning on all of these drugs.
“So, Vioxx was prematurely taken off the market because we really didn't have all the data at that time,” Register says.
Another company called Tremeau Pharmaceuticals is also developing the drug — known generically as rofecoxib — for hemophilic arthropathy, a painful joint disease found in patients with hemophilia.
As the industry seeks to reclaim the drug’s promise, critical questions remain, such as: Can BriOri BioTech get the funding it needs to bring the drug to market? And will Vioxx’s history hinder its marketability when it does?
Here, Register discusses Relÿva, one of the topicals the company is reformulating from existing NSAID medications.
PharmaVoice: When did you begin developing this product?
Bruce Register: Believe it or not, I started this in my garage. That was about three years ago. I think my first experiment was in August of 2019. To make a long story short, after about three months, I had a very good ointment. And we had filed the patent, which was just granted on March 1 of this year.
How has the product performed in pre-clinical research?
When we did human skin diffusion data, we found that we were getting more, but not significantly more, product through the human skin and cadaver skin studies than Voltaren and Pennsaid — the two diclofenac-based competitor products.
Then, in mini-pig studies we got even better results than we were expecting. What we found was we have almost an active transport of our product through the skin. And importantly, we are filing a second patent right now. We found that one of the excipients will increase the amount of rofecoxib directed to the synovial fluid by 60-fold over the oral. And that was a real shocker to us. Now this data was generated not by our company, but by Sinclair Research, which is where the animal work was done, and KCAS in Shawnee, Kansas, which is where the analysis of the plasma and the synovial fluid was done. So, we're very excited about this.
What is the next step for you in this process?
There are two paths we can go down. The one that we're hoping to go with is to get through a phase 1 and phase 2 clinical trial that will be done in 300 patients. We’ll first do a phase 1 trial with about 60 patients in two weeks, to determine the right ointment level. And then the larger trial will be a full-phase 2/3 trial that would be required by the FDA as one of the two pivotal trials to get it approved as a topical.
What people do not realize is that FDA never took [Vioxx] off the market. If you look on the FDA’s Orange Book, it’s listed as discontinued by the manufacturer. So, you can go down a 505(b)(2) pathway [which allows manufacturers of certain types of drugs to acquire FDA approval without going through all the steps required for a New Drug Application].
Does using a topical formulation alleviate some of the safety concerns associated with the oral medication?
Absolutely. [An investigation] found that NSAIDs have a dose effect. So, in other words, the stronger the dose, the higher the level that you have in the system, the more chances of cardiovascular events. So, the lower the systemic exposure — in our case, it will probably be around 10% systemic exposure in comparison to the lowest-approved dose of rofecoxib, which is 12.5 milligrams. What that means is [our topical] will be significantly safer than taking an oral NSAID, even safer than taking ibuprofen or a naproxen, which is over the counter.
Are you concerned about potential marketing challenges related to this product, given Vioxx’s history?
Well, interestingly enough, as I've gone around and talked to people, it's only people that are in their late 50s, 60s and 70s that even remember Vioxx. Those that do, don't care. They just want it back because it helped so much. Almost everybody else in their 20s, 30s, 40s and into the 50s don't ever remember it.
We want to get out in front of the story, instead of coming up behind it. Hopefully that will help and it will also raise awareness among our potential funders to help them understand what it is that we're doing and why we're doing it. We are all doing this because it's been a passion for the last 20 years. We wanted to bring this drug back. I need to do it because I know how many people it’s going to help.