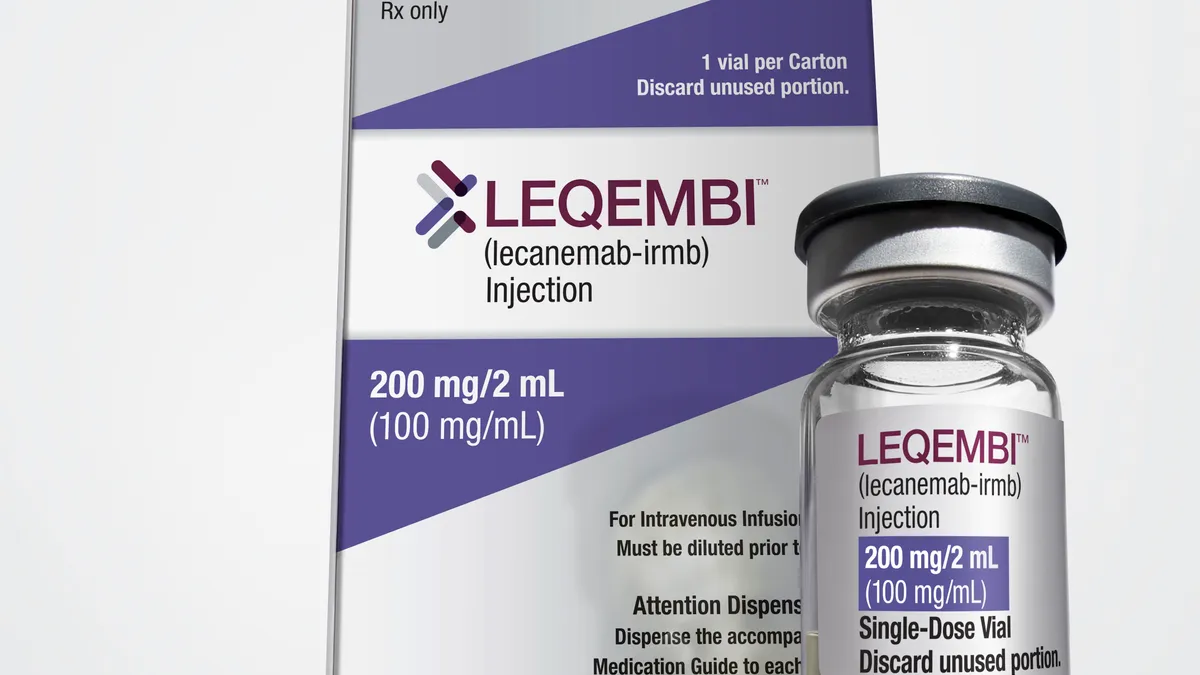
After a slew of twists and turns over the past several years, it looks like Eisai and Biogen finally did it — U.S. regulators gave their Alzheimer’s drug Leqembi (also known as lecanemab) full approval yesterday, setting the treatment up for widespread coverage and patient use, assuming all goes well from here.
The beleaguered duo needed a win, after all. Their previous attempt at a disease-modifying drug for Alzheimer’s — the amyloid plaque-clearing drug Aduhelm, which gained accelerated approval from the FDA in June 2021 — blew up in a series of muddy trials, failed advisory committee votes and denied payer coverage, not to mention a congressional investigation that revealed “irregularities” in the approval process. Biogen and Eisai slashed the marketing for Aduhelm and terminated a real-world clinical trial supplementing the conditional approval, sealing the drug’s fate as a lost cause.
The much smoother road to full approval for Leqembi comes as a breath of fresh air not only for the drugmakers, but for patients and physicians who have awaited a pharmaceutical option for decades. Now that the doors have been opened for regulatory success, other disease-modifying therapies are also poised to take the next step. Out of the 141 unique treatments targeting Alzheimer’s disease in the clinic, here’s a look at the biologic candidates in late-stage trials that could soon make their mark on the therapeutic landscape alongside Leqembi.
The amyloid plaque approach
Both Leqembi and Aduhelm are biologic drugs designed to clear amyloid plaques in the brain that are associated with Alzheimer’s disease and the memory loss that comes with it. A few other drug candidates in phase 3 clinical trials employ the same strategy, though it has been a rocky road to demonstrate the effectiveness of the amyloid theory, and the field has thinned over time.
The company most closely on the heels of Leqembi is pharma giant Eli Lilly, which has been studying the amyloid plaque-clearing treatment donanemab in the clinic since 2013. Late last year, Lilly announced that the candidate removed four times more plaque than Aduhelm in an ongoing head-to-head phase 3 trial expected to end in 2024. Positive results published in May from another late-stage study showed that donanemab slowed cognitive decline, and researchers celebrated the news.
Still, donanemab hasn’t been without its regulatory foibles. The FDA in January denied Lilly’s application for accelerated approval due to a lack of safety data, putting donanemab at a disadvantage compared with Leqembi’s fast-track timeline. Based on the recent study results, however, the company said it planned to apply for full approval by the end of the second quarter.
Donanemab is Lilly’s most advanced candidate after a ten-year trial of another drug called solanezumab failed in March to show a benefit to Alzheimer’s patients. But Lilly has one more trick up its sleeve with the amyloid-clearing treatment remternetug, which showed significant plaque removal in an early-stage trial and is currently in phase 3 with results expected in 2025.
The only other amyloid-clearing candidate carried through to late-stage trials has already been sent back to the drawing board. Roche and its subsidiary Genentech have taken a lot of hits in the Alzheimer’s space, but they haven’t thrown in the towel quite yet. The last straw for the original formulation of their drug candidate gantenerumab was a phase 3 failure announced in November — the treatment cleared much less plaque than expected, and Roche and Genentech halted further trials.
But the Swiss pharma giant is still holding out hope for a new formulation of gantenerumab that it calls trontinemab — this version is designed to cross the blood-brain barrier more easily, theoretically making it more available to clear amyloid plaques.
A shot at tau
Although other companies are attempting to compete with drugs that remove tau tangles from the brain, one biologic stands out in late-stage clinical trials: Eisai’s E2814.
The Japanese company began enrolling a phase 2/3 study of its tau antibody last year with the anti-amyloid drug Leqembi as a background therapy.
Despite 15 years and more than 30 drugs targeting tau aggregation in the brain, none have shown significant efficacy in patients with Alzheimer’s. However, it remains a popular target even though it has taken a back seat to amyloid. Other companies with candidates in early to mid-stage tau trials include Biogen, Bristol Myers Squibb, Johnson & Johnson, Lilly, AbbVie, Roche, Merck and more.
Besides amyloid and tau, two more phase 3 candidates could have an impact on the treatment landscape for Alzheimer’s.
One is the Korea-approved pancreatic cancer vaccine tertomotide from GemVax and Kael Bio. The treatment stands apart from the others in advanced trials as an immunotherapy-based approach, and phase 3 results from a study begun last year are expected in 2026.
Finally, the immensely popular weight loss drug semaglutide from Novo Nordisk — known as Ozempic, Wegovy and Rybelsus at different doses — could play a part in early Alzheimer’s by improving nerve cells, inflammation and vascular health, according to Alzheimer’s Disease International.