Party like it’s 1959 — Barbiemania is back. But society looks a whole lot different today than it did when Mattel first introduced the doll, particularly for women. And in no area is this shift more noticeable than healthcare.
Just think — when Barbie hit the market in the spring of 1959, oral contraceptives weren’t available yet and wouldn’t be for another year. At the time, women also were rarely, if ever, included in clinical trials.
Conditions didn’t start improving until the early 1990s (around the time the bestselling Barbie was released), when the FDA mandated inclusion of women in NIH-sponsored studies, and soon after established the Office of Women’s Health.
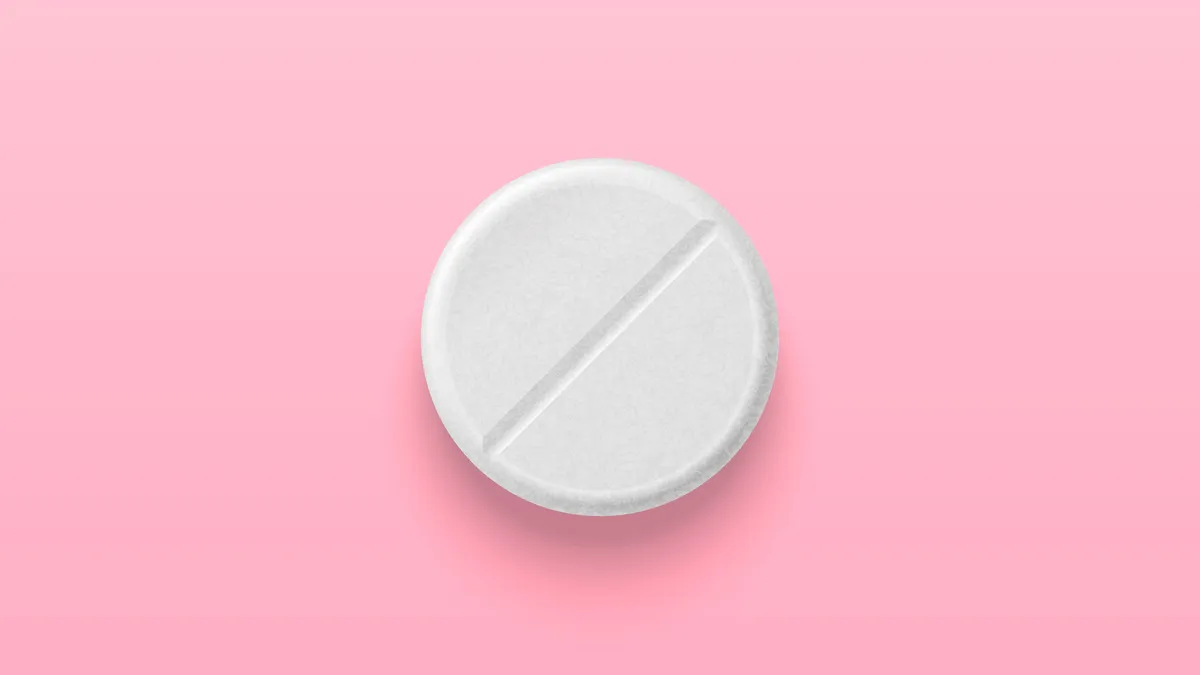
Fast-forward to today, and as the doll hits the silver screen in a newly released movie, investment in women’s healthcare is hotter than ever. Just this month, the FDA approved the first over-the-counter birth control pill, further expanding women’s reproductive freedom. The regulator also gave the OK earlier this year to the first nonhormonal treatment for a common symptom of menopause.
Of course, there’s still a long way to go. And as the world turns pink for Barbie, we’re looking at recent strides in the women’s health landscape and the steps needed in the future.