How SaMD benefits pharma, patients, and clinicians, and how to get it right
Author: Matt Norton, Vice President of Marketing & Commercial Development, S3 Connected Health
Pharma companies are now developing more Software as a Medical Device (SaMD) as they present the best opportunities to expand their offering and broaden the value they can bring with digital health solutions.
Yet, while pharma companies are familiar with the concept of digital health solutions, it’s important to be clear that not all digital health solutions are SaMD.
Put simply, SaMD is “software intended to be used for one or more medical purposes that perform these purposes without being part of a hardware medical device.” Not included within this definition is software with the intended use of driving a hardware medical device, or those that simply fetch data.
Success with these kinds of solutions depends on pharma’s ability to challenge traditional thinking, and recognize the ways in which developing SaMD solutions can not only complement existing drugs and therapies, but improve insight, adherence, and outcomes, too.
Here’s why pharma companies should be aware of and embrace SaMD digital health solutions, and how to go about it.
How SaMD solutions are different, and why they’re on the rise
The key difference is the stricter regulations to which SaMD solutions are subject. As the clinical claims associated with a SaMD solution increase, so does the evidence pharma companies must provide to support their claims, and the stringency with which the software is regulated.
Less strictly regulated digital health solutions that aren’t classified as SaMD can still add value when it comes to patient education and onboarding. But SaMD solutions can allow pharma to address a much wider range of challenges and differentiate themselves more clearly in the market.
To really maximize benefits for patients, clinicians, and other stakeholders, pharma companies need to embrace SaMD solutions.
What are the benefits SaMD solutions?
The stricter regulatory burden and increased complexity mean that SaMD solutions take longer to develop. But the potential value and return for that investment makes it more than worthwhile: the first key step is aligning internal stakeholders on that potential value to get projects up and running with the correct backing.
So how can pharma companies, clinicians, and patients benefit from SaMD solutions in practice?
Compared to unclassified digital health applications, SaMD solutions often play a more direct role in the care of an individual; they’re more likely to impact patient outcomes as a result.
Many SaMD solutions support holistic management of conditions by helping to manage symptoms and comorbidities. While this has obvious benefits for patients, pharma companies developing these solutions can often differentiate themselves and champion treatment for a given disease.
Existing pharmaceutical treatments can also be differentiated by combining them with digital solutions that have a demonstrable influence on real-world outcomes, creating a far more effective treatment plan than one that uses drugs alone.
What’s more, strict control and regulation can lead to increasing confidence among clinicians, and thus increase adoption as a result when compared with non-regulated digital health options.
Capturing standardized, real-world data and evidence with SaMD solutions allows for insights to be generated on treatment of diseases, their trajectory and development. SaMD solutions can also support reimbursement with real-world evidence, subsequently improving engagement with healthcare providers as well as payors, and ensuring the costs of patient treatment are quickly covered.
Digital therapeutics are often SaMD solutions, but there are others
DTx and SaMD are not synonymous, but the majority of digital therapeutics (DTx) are classified as SaMD.
DTx provide software-driven therapeutic interventions for the treatment, management, or prevention of a disease or disorder. They function in three key ways: either combining with an existing treatment, augmenting a treatment, or being prescribed as a standalone treatment.
The reason DTx solutions usually fall into the SaMD category is because they play a more active role in treatment and management of diseases, and so require clinical evidence to support product claims. Their efficacy and safety claims must also be subject to regulatory approval and oversight.
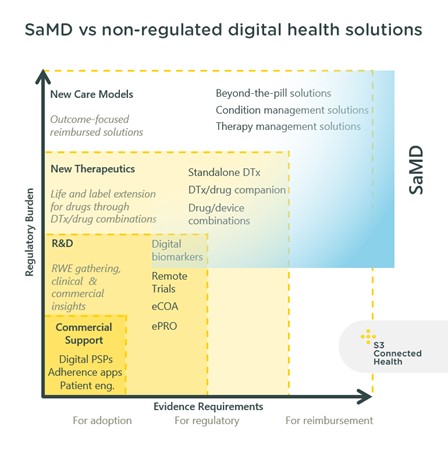
The diagram above maps out digital health solutions, with evidence requirements and regulatory burden increasing along each axis. Most of the solutions listed in the blue section will be regulated as SaMD; while DTx are included in this category, there are other types of SaMD solutions.
Welldoc is an example of a DTx that is regulated as SaMD. Through their digital health platform, the company offers several products in the diabetes and cardiology space; they’re also a member of the Digital Therapeutics Alliance. Vinehealth Cancer Companion is a Class I SaMD which is not a DTX. The mobile application supports patients through their cancer treatment by helping them track, manage, and understand their treatment but does not directly influence outcomes.
DTx are one of the more well-defined types of digital health solutions out there, and so they offer tangible examples of the benefits of SaMD for pharma. These more strictly regulated DTx place pharma companies at the forefront of innovation in new therapeutic categories, generating new revenue streams and/or business models by extending existing portfolios, or entering new therapy areas. The clinical evidence and regulatory oversight these solutions are subject to have the added benefit of increasing patient and clinician confidence.
Clinicians can provide and receive oversight and information on treatment progress with data insights, support treatment decisions with robust clinical evidence, and prescribe DTx in regions with regulatory approval and reimbursement options.
SaMD solutions are more complex, but worthwhile
There’s no doubt that creating SaMD solutions is a bit more difficult, especially for pharma companies that have never done it before. But while there’s more to consider to get it right, there are countless benefits to doing so.
What’s more, pharma doesn’t need to go down the long road of developing SaMD-specific regulatory and development skills in-house to harness the benefits. A specialist digital health firm like S3 Connected Health can not only offer better value in the long run but it can help pharma companies avoid the costly mistakes of a trial-and-error, in-house approach.
We’ve seen more pharma companies develop SaMD solutions in recent years, but we need to continue this trend upwards. By following this trajectory, pharma can have a bigger impact on outcomes, maximise the value of their existing offerings, and arm patients and clinicians with the resources and tools they need in a world of increasingly digital and personalized healthcare.
Securing the benefits of SaMD solutions means finding the right capabilities to succeed. But with trusted partners on hand to assist with the regulatory burden of SaMD – and much more besides – there’s every reason for pharma to succeed.
