Page 2
-
Teen’s death following Sarepta DMD gene therapy underscores a risk seen for decades
The death of a 16-year-old boy evokes a lengthy battle to realize the benefit of cutting-edge gene therapy while also enduring tragic risk.
-
The key factors shaping China’s biopharma boom
Chinese companies are involved in one-fifth of pharma’s clinical development programs, but trade wars and regulatory hurdles could stymie dealmaking.
-
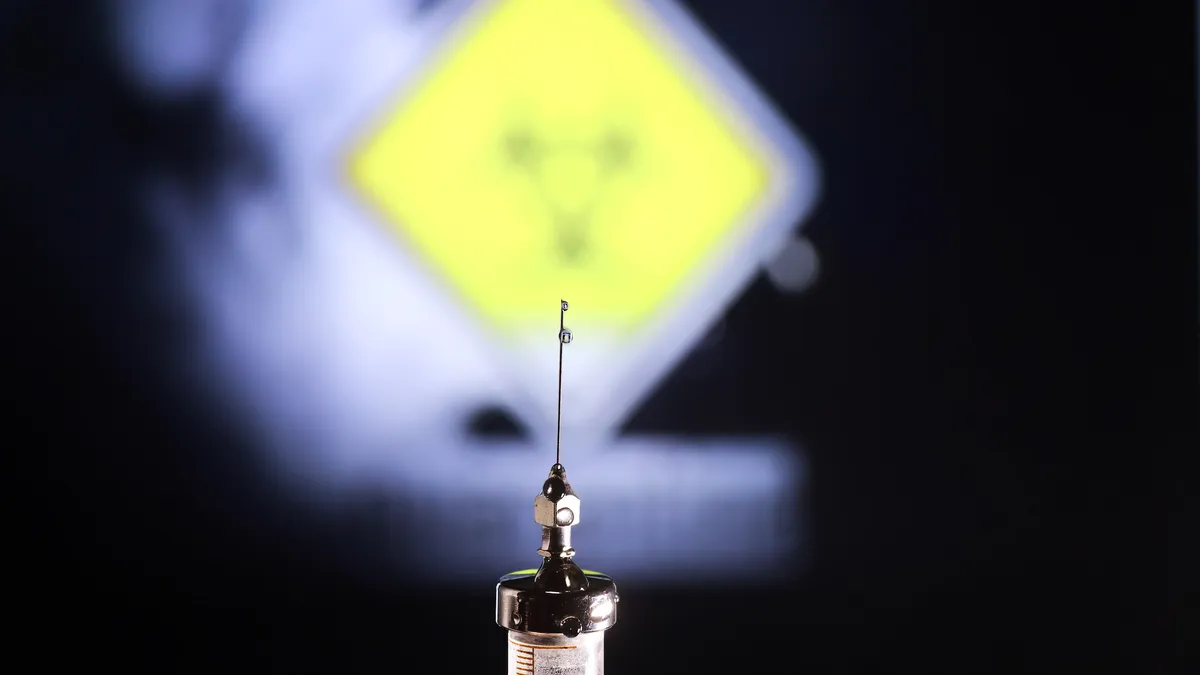
Vaccine makers face mRNA backlash as states seek further restrictions
A handful of states are targeting the technology at the same time the Trump administration is cutting research funding and revoking vaccine contracts.
-
Q&A
How non-traditional investors fuel up-and-coming biotechs when VC funding dries up
Smaller biotechs are finding creative ways to fund R&D at a time when the traditional financing channels have become more constrictive.
-
Drug developers ‘haven’t even scratched the surface’ of what radiopharma can do
Even with Big Pharmas pouring billions into the space, the potential of radiopharmaceuticals remains largely untapped.
-
Q&A
Roche’s obesity deal with Zealand Pharma tees up rival for GLP-1s
The new $5.3 billion deal with the Swiss pharma giant gives Zealand a significant partner to advance its amylin analog obesity shot and create a new combination product.
To find more content, use the "Topics" in the menu above.